Ever wondered about the potent liquid used in everything from cleaning pools to refining metals? We're talking about hydrochloric acid (HCl), a powerful chemical with a fascinating history and a wide range of applications. This guide delves into the methods of hydrochloric acid synthesis, exploring the process from its intriguing origins to its modern-day uses. But beware, this isn't a kitchen chemistry experiment; generating HCl demands respect and careful adherence to safety protocols.
Generating hydrochloric acid is not something to be undertaken lightly. It requires a deep understanding of the chemical reactions involved and meticulous attention to safety. While information on HCl production is readily available, actually producing it requires specialized equipment and a controlled environment. This isn't your average DIY project; it's a serious endeavor with significant risks if not handled correctly. So, before you even think about donning your lab coat, make sure you understand the gravity of the undertaking.
The history of hydrochloric acid production is intertwined with the very development of chemistry itself. Early alchemists stumbled upon its corrosive power, recognizing its potential but also its dangers. Over the centuries, methods for producing HCl evolved, moving from crude and often hazardous techniques to the more refined processes employed today. Understanding this history gives us a greater appreciation for the complexity and potential hazards associated with HCl synthesis.
Producing hydrochloric acid has significant implications across various industries. From its role in producing PVC plastics to its use in the food industry, HCl is a crucial component in countless processes. However, the production of hydrochloric acid can also pose significant environmental challenges. Responsible HCl generation requires careful management of byproducts and waste to minimize ecological impact. The responsible production of HCl is not just about maximizing yield; it's about minimizing environmental harm.
So, what are the primary concerns when synthesizing hydrochloric acid? Safety, safety, and safety. Handling concentrated HCl demands rigorous adherence to safety protocols, including proper ventilation, protective gear, and a thorough understanding of the chemical reactions involved. Incorrect handling can lead to severe burns, respiratory problems, and other serious health issues. Before even considering producing HCl, educate yourself on the necessary safety measures.
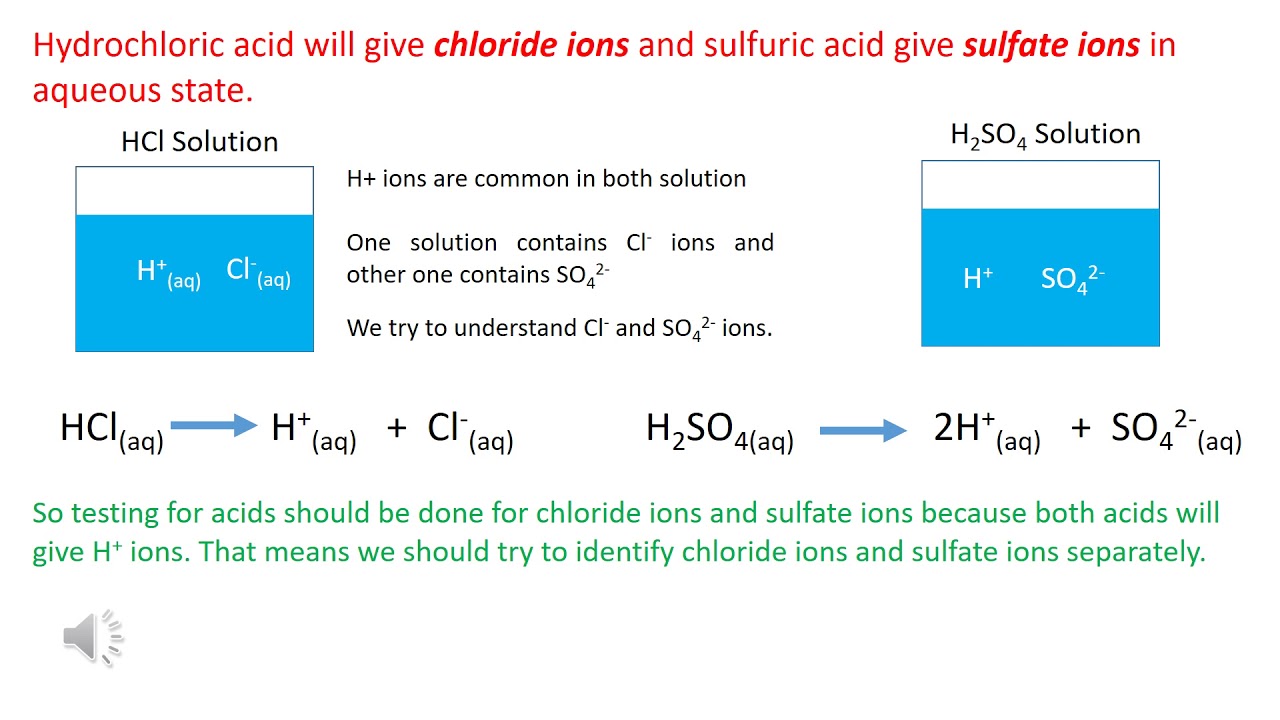
Hydrochloric acid is formed by dissolving hydrogen chloride gas in water. This process is exothermic, meaning it releases heat. One simple way to visualize this is by imagining the hydrogen chloride gas molecules interacting with water molecules, releasing energy in the form of heat.
While creating HCl is generally not recommended for home experiments due to safety concerns, understanding its production theoretically can be educational. However, practically producing it should be left to industrial settings with appropriate safety equipment and trained personnel.
Advantages and Disadvantages of HCl Production
| Advantages | Disadvantages |
|---|---|
| Essential for various industrial processes | Highly corrosive and dangerous if mishandled |
| Relatively inexpensive to produce in industrial settings | Production can generate harmful byproducts |
Producing hydrochloric acid requires strict safety procedures, appropriate equipment, and a controlled environment. It's highly recommended to leave HCl production to industrial facilities equipped to handle the associated risks.
Frequently Asked Questions:
1. What is hydrochloric acid? - A strong corrosive acid.
2. Is making HCl dangerous? - Yes, extremely dangerous without proper precautions.
3. Can I make HCl at home? - It's strongly discouraged due to safety risks.
4. What are the uses of HCl? - Various industrial processes, including metal cleaning and food production.
5. How is HCl produced industrially? - Primarily through the reaction of hydrogen and chlorine gas.
6. What are the safety precautions for handling HCl? - Proper ventilation, protective gear, and a thorough understanding of its properties.
7. What should I do if I spill HCl? - Consult safety data sheets and seek professional assistance.
8. Where can I find more information about HCl? - Reputable scientific websites and chemical textbooks.
In conclusion, while understanding the process of hydrochloric acid production can be intellectually stimulating, actually creating it outside of a controlled industrial setting is highly discouraged. The risks associated with handling concentrated HCl are significant, requiring specialized knowledge, equipment, and meticulous attention to safety protocols. While HCl plays a vital role in numerous industries, generating it at home is simply not worth the risk. For those interested in learning more, consult reputable scientific resources and prioritize safety above all else. Remember, knowledge is power, but safety is paramount. Explore the fascinating world of chemistry responsibly, and leave the production of HCl to the professionals.
Conquer your nj dmv title change appointment guide
Sweet molasses behr paint a rich warm hue for your home
Unlocking your inner emo aesthetic gacha online outfit inspiration